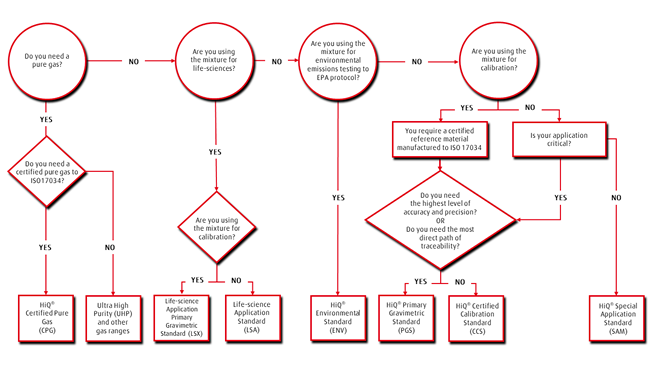
- Is your mixture used for the calibration of an instrument?
- If yes, you will need a certified reference standard such as BOC’s HiQ® Primary Gravimetric Standard (PGS) or Certified Calibration Standard (CCS). Both are compliant to ISO 17034 and are fully traceable. PGS mixtures are certified gravimetrically with analytical verification and overall provide the tightest preparation tolerances and smallest certification uncertainty, compared to CCS mixtures which are certified analytically. Such standards can be used to calibrate analytical equipment which is used in research, production laboratories and process monitoring. For example, for analysis of LNG for export where 0.5% difference in calibration uncertainty has a significant financial impact on the fiscal risk of the product, a PGS mixture may be more appropriate. Both PGS and CCS mixture types can accommodate your complex multi-component mixtures with an extensive, ever expanding range of gases and liquids.
- Do you have a critical process application which relies heavily on accurate concentrations and guaranteed stability?
- If your mixture is not used for calibration, but you have a critical process application which heavily relies on the mixture to provide precise and accurate concentrations with guaranteed stability over its entire shelf-life, then consider using one our HiQ ® Certified Calibration Standards (CCS). Otherwise, for simple mixtures comprising 4 components or less, required for applications such as animal cell culture, blanketing, instrumentation support gases and leak detection, a HiQ® Special Application Mixture (SAM) may provide a more cost-effective option. This is especially the case for high usage. SAM mixtures are volumetrically or gravimetrically prepared and can be supplied with or without a cylinder-specific NATA-accredited Certificate of Analysis to ISO 17025.
- Are you performing a life science application?
- BOC offers dedicated products for life science applications. If you are using mixtures in life science applications such as in IVF clinics, hospitals or universities and you are calibrating instruments, then you will need a HiQ® Life Science Application Mixture Plus (LSX). This certified reference material is manufactured in accordance with ISO 17034 and under Good Manufacturing Practice (GMP). Alternatively, a more cost-effective solution could be HiQ® Life Science Application (LSA) mixtures which are suitable for non-calibration purposes and produced under GMP.